Avondlezing door Giulia Finotello en Xiaocheng Mi georganiseerd door de Groningse Chemische Kring.
Iron as energy carrier: the iron oxidation/reduction cycle
Samenvatting presentaties
Dr. G. Finotello
Prospects and challenges of the iron oxide reduction for the iron fuel cycle
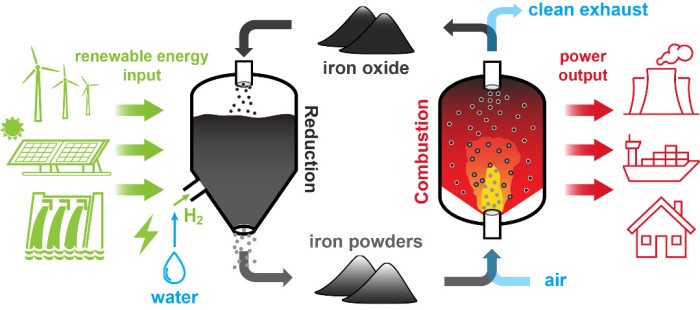
The iron fuel cycle produce solid iron-oxide combustion products that can be easily separated and then be stored for later recycling. Unfortunately, Many current metal production and recycling technologies rely heavily on fossil energy inputs. However, at Eindhoven University of Technology we are exploring two zero-carbon reduction processes: the reduction with hydrogen in fluidized beds and the electrochemical reduction.
Hydrogen can be used to directly reduce iron oxide to iron, enabling zero-carbon metal-fuel production using industrial-scale technologies that are commercially available such as fluidized bed reactors. In the lab, the fluidization and reduction behaviour of combusted iron powder is studied in a fluidized bed. We observed that the measured minimum fluidization velocity starts to deviate from the Ergun correlation at temperatures above 340 ⁰C. We therefore extended the Ergun equation by considering the cohesive solid bridge force to better predict the umf at elevated temperatures. The sticking of particles and the formation of a dense layer of wüstite severely limit the reaction rate, posing a challenge to the reduction reaction at temperatures higher than 600 ⁰C. The reduction process also alters particle properties such as morphology and density, affecting the high-temperature fluidization characteristics and cyclic use of particles.
Direct production of metals using electricity is possible through electrochemical reactions, commonly known as electrowinning. Proof-of-concept experiments are performed using a single parallel plate electrode, immersed in a mixture of micro-sized hematite (Fe2O3) powder and strong aqueous alkaline (NaOH, 50%wt, 18 M) electrolyte. A cathodic deposition of metallic iron with a high Faradaic efficiency (> 90%) is successfully accomplished. Nevertheless, high and reproducible Faradaic efficiencies have been achieved mostly when using pure iron oxides as a feedstock rather than combusted iron.
In the future, other routes such as reduction of iron oxides in hydrogen flame and reduction in hydrogen plasma will be considered.
Dr. XiaoCheng Mi
Combustion of iron powder: From fundamental to applications
The true reason underlying the energy crisis faced by our society is not the lack of renewables sources, but the mismatch between a continuous energy demand and a geographically scattered and temporally intermittent supply from these sources. To overcome this problem, the scientific community is in search of reliable and efficient energy storage alternatives. Metal-enabled Cycle of Renewable Energy (MeCRE) has been proposed as a solution for sustainable, long-distance transport and long-term storage of clean energy. Owing to its high energy density, zero-carbon nature, and recyclability, iron powder is nowadays considered as the most promising energy carrier to realize MeCRE on a global scale. To build real-world power and heat generation systems, fundamental insights into the combustion properties of fine iron particles are required. Although a decent amount of knowledge in the combustion of conventional solid fuels is useful, numerous unique questions rooted in iron-powder combustion must be answered. These questions are mainly related to two unique features of iron-powder combustion: (I) The heterogeneous oxidation mechanism of individual iron particles and (II) the resulting laminar and turbulent flame dynamics with a spatially discrete heat release.
This lecture presents an overview of some current efforts made towards answering several outstanding questions in iron-powder combustion using multiphysics modeling approaches. A single-particle combustion model informed by molecular dynamics (MD) simulations is used to determine the rate-limiting mechanism of the oxidation of a liquid iron droplet at elevated temperatures. This study reveals that the oxidation rate of an iron droplet is not solely controlled by the external diffusion of O2, but an interplay among external diffusion, surface absorption, and internal transport. Euler-Lagrange CFD simulations are used to answer the question as to how the spatially non-uniform energy release from iron particles influences the flame dynamics under application-relevant conditions. Further challenges that we must overcome to develop practical iron-powder combustors are summarized.
Loopbaan Dr. Giulia Finotello After completing her studies in Chemical Engineering at the Polytechnic of Torino (Italy) and an internship at the chemical company BASF in Ludwigshafen (Germany), Giulia Finotello started her PhD at the Chemical Engineering Department of Eindhoven University of Technology (TU/e). In February 2019, she obtained her PhD with a thesis on Droplet collision dynamics in a spray dryer-experiments and simulations under the supervision of Professor J.A.M Kuipers of the Multiphase Reactors Group. Giulia worked during her postdoc on the design and teaching of a new course, Practical Transport Phenomena, for the Bachelor program of Chemical Engineering. From October 2019, she is assistant professor in the Power and Flow Section of the Mechanical Engineering Department of TU/e. Her research focuses on the production and use of metal powder for additive manufacturing and the regeneration of metal powders as part of the metal fuel cycle. She received the award for best oral presentation at the 13th International Conference on Computational Fluid Dynamics in the Minerals and Process Industries in 2018 and the award for best teacher for Bachelor Mechanical Engineering in 2021. She is also an organizer of the 1st Workshop on Image is everything: Expanding the capabilities of particle imaging technology at theLorentz Center, Leiden September 2022 and the 1st Workshop on Metal-enabled Cycle of Renewable Energy (MeCRE) at TU/e in November 2022.
After completing her studies in Chemical Engineering at the Polytechnic of Torino (Italy) and an internship at the chemical company BASF in Ludwigshafen (Germany), Giulia Finotello started her PhD at the Chemical Engineering Department of Eindhoven University of Technology (TU/e). In February 2019, she obtained her PhD with a thesis on Droplet collision dynamics in a spray dryer-experiments and simulations under the supervision of Professor J.A.M Kuipers of the Multiphase Reactors Group. Giulia worked during her postdoc on the design and teaching of a new course, Practical Transport Phenomena, for the Bachelor program of Chemical Engineering. From October 2019, she is assistant professor in the Power and Flow Section of the Mechanical Engineering Department of TU/e. Her research focuses on the production and use of metal powder for additive manufacturing and the regeneration of metal powders as part of the metal fuel cycle. She received the award for best oral presentation at the 13th International Conference on Computational Fluid Dynamics in the Minerals and Process Industries in 2018 and the award for best teacher for Bachelor Mechanical Engineering in 2021. She is also an organizer of the 1st Workshop on Image is everything: Expanding the capabilities of particle imaging technology at theLorentz Center, Leiden September 2022 and the 1st Workshop on Metal-enabled Cycle of Renewable Energy (MeCRE) at TU/e in November 2022.
Loopbaan Dr. XiaoCheng Mi XiaoCheng Mi is an assistant professor in the Power and Flow Section, Department of Mechanical Engineering, Eindhoven University of Technology (TU/e). He received his PhD degree in Mechanical Engineering at McGill University (Canada) in May 2018 under the supervision of Prof. Andrew J. Higgins. His doctoral dissertation is on "Detonation in spatially inhomogeneous media". From Aug. 2018 to Feb. 2020, he was supported by the Canadian NSERC Postdoctoral Fellowship to pursue a postdoctoral study on detonation of multiphase energetic materials at the Centre for Scientific Computing (CSC), Cavendish Laboratory, University of Cambridge (UK). From Mar. 2020 to Oct. 2021, XiaoCheng did his second postdoctoral research on metal combustion at the Alternative Fuel Laboratory (AFL), McGill University, directed by Prof. Jeffrey M. Bergthorson. He received the John H.S. Lee Young Investigator Award at the 27th International Colloquium for Dynamics of Explosions and Reactive Systems (ICDERS) in 2019. He is also an organizer of the Young Researchers’ Forum on Detonation (YRF-Det) and the 1st Workshop on Metal-enabled Cycle of Renewable Energy (MeCRE) at TU/e in November 2022.
XiaoCheng Mi is an assistant professor in the Power and Flow Section, Department of Mechanical Engineering, Eindhoven University of Technology (TU/e). He received his PhD degree in Mechanical Engineering at McGill University (Canada) in May 2018 under the supervision of Prof. Andrew J. Higgins. His doctoral dissertation is on "Detonation in spatially inhomogeneous media". From Aug. 2018 to Feb. 2020, he was supported by the Canadian NSERC Postdoctoral Fellowship to pursue a postdoctoral study on detonation of multiphase energetic materials at the Centre for Scientific Computing (CSC), Cavendish Laboratory, University of Cambridge (UK). From Mar. 2020 to Oct. 2021, XiaoCheng did his second postdoctoral research on metal combustion at the Alternative Fuel Laboratory (AFL), McGill University, directed by Prof. Jeffrey M. Bergthorson. He received the John H.S. Lee Young Investigator Award at the 27th International Colloquium for Dynamics of Explosions and Reactive Systems (ICDERS) in 2019. He is also an organizer of the Young Researchers’ Forum on Detonation (YRF-Det) and the 1st Workshop on Metal-enabled Cycle of Renewable Energy (MeCRE) at TU/e in November 2022.
Introducé(e)s zijn van harte welkom.
Graag vooraf bericht als u verwacht te komen.
Stuur daartoe een mail naar gck@kncv.nl of kommer.brunt@planet.nl.